What is ATP?
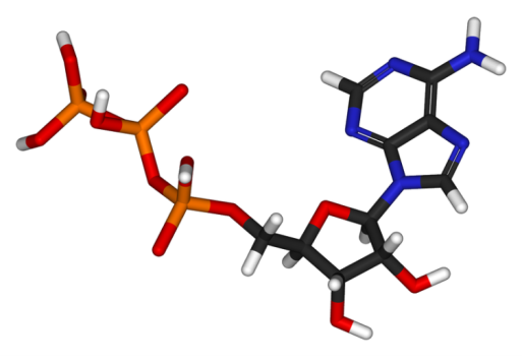
Adenosine triphosphate (ATP) is a molecule used by all living cells to provide energy to metabolic reactions. It is often referred to as the “molecular unit of currency” of intracellular energy transfer. As ATP is specific to living environments, its presence proves the existence of living organisms.
ATP testing is a technique measuring the level of ATP in a sample in just a few minutes.
There are two forms:
- Free or extracellular ATP,
- Intracellular ATP.

Free ATP
It is the ATP freed by the dying or dead cells. When a cell dies, it loses its membrane integrity. The ATP, which is a very small molecule, is released in the environment.
As it is an unstable molecule in unbuffered water, it is rapidly destroyed. Its stability depends on many parameters:
- pH: free ATP is stable at neutral pH but its degradation rate quickly increases with alkaline and acidic pH.
- Temperature: degradation rate increases with temperature. Rather stable at 4°C, it degrades quickly at 25-30°C.
- Presence of stabilisers: such as polycations and/or some cations.
- Type of biocide used: oxidising biocides such as chlorine or bromine rapidly degrades free ATP whereas non oxidising biocides have little effect.
- Presence of other microorganisms: some are able to retrieve free ATP to use it.
Therefore, free ATP stability is difficult to assess because it depends on many parameters. Indeed, if the environment provides a good stability, free ATP will accumulate. However, if the environment is unfavorable, free ATP will disappear very quickly.
Intracellular ATP
It is the ATP found in the living cells. As mentioned above, this molecule plays a vital role in intracellular energy transfer. It is permanently renewed and recycled in the cell, but its production immediately stops when the cell dies.
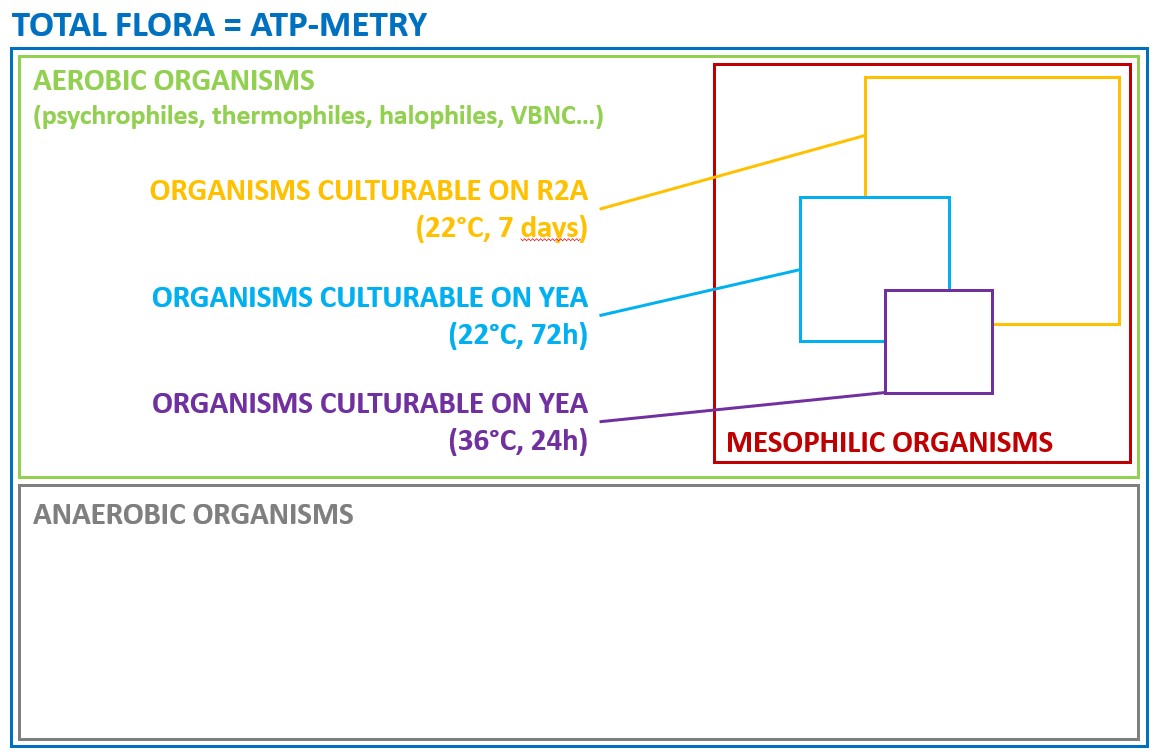
Total ATP is defined as the sum of free ATP and intracellular ATP. To measure it, it is necessary to destroy the cells with a lysis buffer in order extract the ATP. The measurement is done on the freed intracellular ATP and the free ATP.
To assess the quantity of microorganisms in a sample, only intracellular ATP must be measured.
“Total ATP – free ATP = intracellular ATP”
A short-sighted and risky approach…
How to measure intracellular ATP?
To quantify intracellular ATP, two different approaches have been developed: an indirect measurement and a direct measurement.
The indirect measurement:
It was the first technique to be developed. It is based on the assumption:
Total ATP – free ATP = intracellular ATP
Intracellular ATP is deduced from the measurement of total and free ATP.
- Measurement of free ATP: it is measured by bioluminescence without lysis buffer. Thus, only extracellular (free) ATP is able to react with the bioluminescence enzyme. However, as described above, the quantity of free ATP varies greatly depending on many factors. It is not reprensentative of the quantity of microorganisms in the sample. Therefore, the measurement uncertainty is high.
- Measurement of total ATP: it is measured by bioluminescence with lysis buffer that destroys the cells. Intracellular ATP is freed and add up to the free ATP. As the volumes of sample are generally low (around 100 µl), the presence of biofilm fragments strongly affects the result.
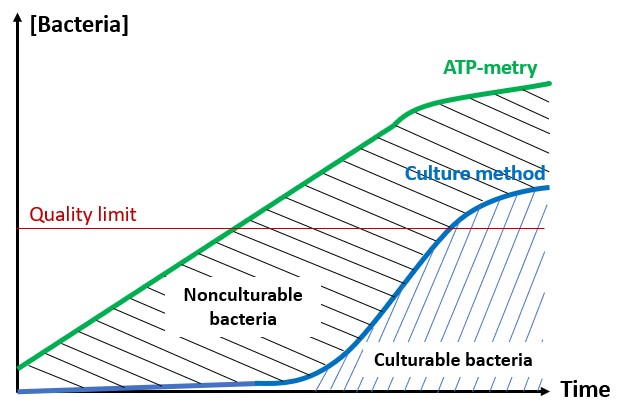
Consequently, using this strategy, the measurement of intracellular ATP is based on the subtraction of two uncertain measurements, which gives an approximate or even a false result.
The other problem lies in the fact that ATP measurement is relative. Indeed, the result is given in Relative Light Unit by the luminometer (RLU). Yet, in this indirect approach, the measurement is not standardised. It depends on many parameters affecting the enzyme efficacy (temperature, effect of lysis buffer, biocides…).
Working on qualitative measurements only leads to important approximations. It is even possible to get higher quantity of free ATP than total ATP!
In conclusion, the measurement of intracellular ATP by this approach is quick and easy. However, given the issues described above, the result can be bias and thus difficult to interpret. Therefore, it is essential to cautiously process the data to avoid over-interpretations.
Direct measurement of intracellular ATP
An extra step is required to measure intracellular ATP directly: filtration on a membrane to eliminate the free ATP. Indeed, as this molecule is very small, it passes through the membrane whereas the microorganisms are retained.
In this approach, the microorganisms retained on the filter are then destroyed in order to free the intracellular ATP. It gives a representative view of the living organisms in the sample.
Besides, this strategy has the advantage of analysing a representative volume of sample (generally between 10 and 50 ml).
Although requiring a little more handling, this approach, combined with standardisation, gives quantitative results much more reliable and comparable in time and space.
Conclusion
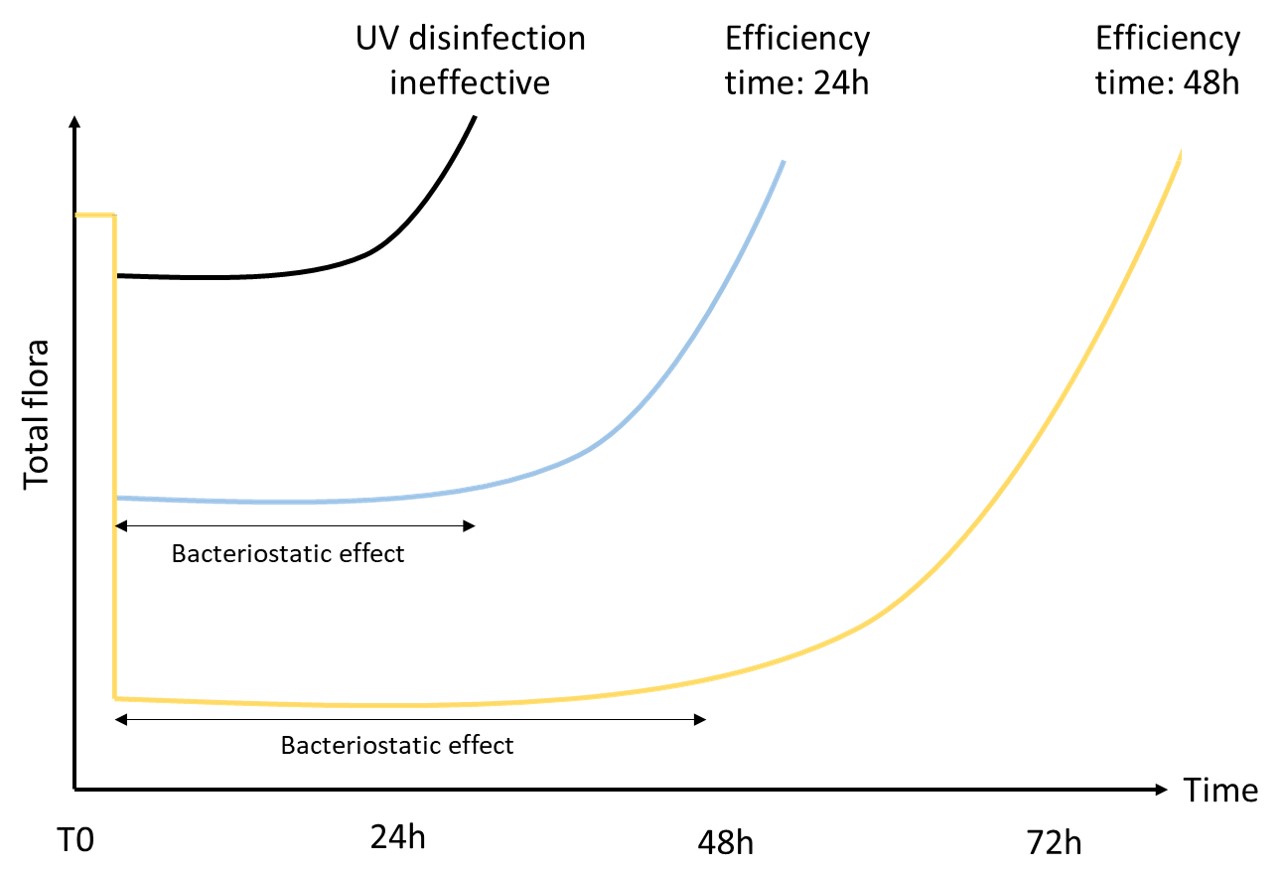
ATP testing is one of the fastest and easiest methods to detect microorganisms in water. This 40-year-old analysis has obviously evolved over time.
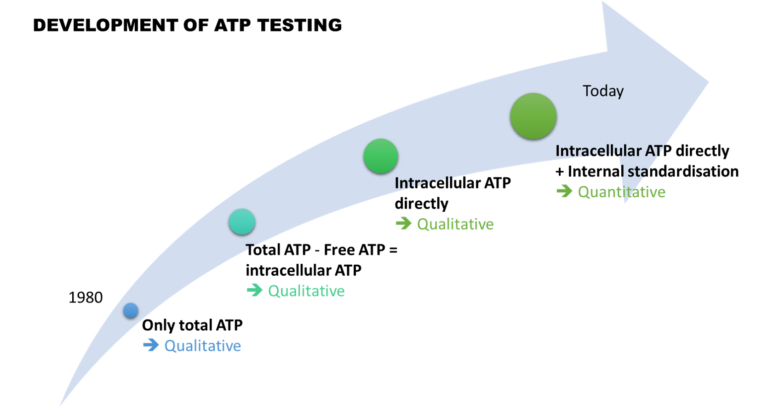
At first, only total ATP was detected qualitatively. Then, thanks to the measurement of free ATP, it was possible to indirectly assess the quantity of intracellular ATP. However, due to high variability, the result interpretation remained complicated.
Then, 15 years ago, new approaches emerged. They now include a filtration step to overcome the problems of variability.
Finally, the integration of external and then internal standardisation made this analysis quantitative. It became robust and comparable in time and space, making ATP testing a relevant analysis.